
Please verify your age before entering the site.

A premarket tobacco application (PMTA) is an application that must be reviewed and approved by the Food and Drug Administration (FDA) before new or currently marketed tobacco products can be legally marketed and sold in the United States. Electronic Nicotine Delivery Systems (ENDS) are subject to the PMTA requirements according to Federal Food, Drug, and Cosmetic Act (FD&C Act) section 910(a). Through a comprehensive science and evidence-based approach, PMTA shall demonstrate that the new tobacco product to be marketed would be appropriate for the protection of the public health (APPH). The following key components must be included in a PMTA submission:

FDA suggests submitting your PMTA based on above documents and reviewing it as required by the PMTA Review Process. Once approved, it can be officially released in the US market.
Learn More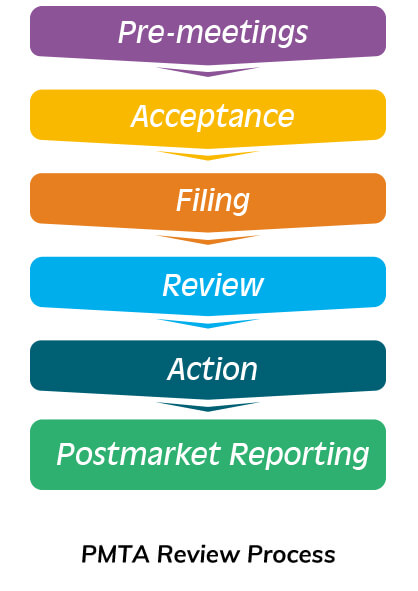
JWEI has a team with experts from various fields including regulations/toxicology/chemistry/clinical research. We also work with FDA-accredited global testing laboratories and the world's leading CROs to ensure full compliance with PMTA regulations.
Prior to performance of a full-scale PMTA study, a Pre-PMTA evaluation is conducted in the early stage through JWEI’s independent laboratories with state-of-the-art metrology and testing capabilities. This pilot test includes E-cigarette chemistry and analytical detection, product safety and UL8139 testing.
We work closely with a number of famous US E-liquid brands. We can pair your device with multiple high-quality E-liquids for PMTA required tests. Meanwhile we can also help any E-liquid manufacturer to develop and refine a cost-effective PMTA strategy for your E-liquid products.
JWEI has extensive experience and expertise in managing all types of complex projects. With a comprehensive one-stop service, we can efficiently transform strategies and solutions into sustainable results and ensure that your project can be completed on time & under budget.
JWEI provides professional automatic design and manufacturing based on your needs. Automation can effectively improve the production capacity and quality, and therefore reduce the lead-time and costs.
JWEI has a complete quality management system with advanced manufacturing and testing capabilities. All products are certified with ISO 9001 quality management system, ISO 14001 environmental management system, GMP820/110, HACCP, and UL8139. JWEI provides customers with a full range of quality assurance and services.